
FDA Gives CryoCor the Cold Shoulder
San Diego-based CryoCor suffered a blow today, when it learned that FDA is yet again unhappy with the company’s pre-market approval application for its Cryoblation […]

San Diego-based CryoCor suffered a blow today, when it learned that FDA is yet again unhappy with the company’s pre-market approval application for its Cryoblation […]

Shelhigh has agreed to stop distributing its implantable devices, roughly two months after FDA seized products made at the company’s Union, NJ, manufacturing facility. Under […]

BioLucent, a Aliso Viejo, CA-based company that makes breast cushions for increased comfort during mammographies, has been scooped up by Hologic, a diagnostic imaging company […]

SyntheMed, of Iselin, NJ, today got word that an FDA panel will advance the first anti-adhesion product in what the company estimates is a $2 […]

Minneapolis-based ATS Medical has agreed to acquire the surgical cryoablation business of CryoCath Technologies, a Canadian company that will now focus exclusively on electrophysiology cryoablation. […]

Cambridge Heart, of Bedford, MA, has amended its marketing agreement with St. Jude Medical in a way that could significantly expand access to tests for […]
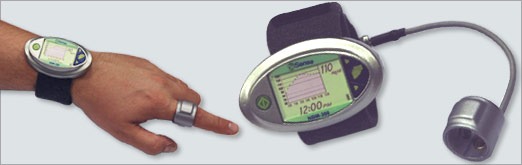
OrSense, a privately held Israeli company, has been cleared in Europe to market its first product, a first-in-class, non-invasive glucose monitor. The NBM-200G allows diabetics […]

Companies developing molecular and specialty diagnostics are poised to outperform other sectors of medical technology in the coming years, according to an article that ran […]

With its final pivotal trial moving along as planned, Irvington, NY-based Electro-Optical Sciences is actively initiating partnering discussions for its point-of-case melanoma-detection device. MelaFind is […]

Small Bone Innovations (SBi), a New York, NY, company focused exclusively on small bone and joint orthopedic devices, has received FDA clearance to market a […]
Copyright © 2026 | WordPress Theme by MH Themes